Introduction
Clinical guidelines recommend initial treatment with OACs, such as direct-acting oral anticoagulants (DOACs; apixaban, dabigatran, edoxaban, rivaroxaban) or warfarin, for at least 3 months after an initial venous thromboembolism (VTE) event. Early OAC use is associated with improved clinical outcomes and helps prevent VTE recurrence.
Purpose
To estimate the proportion of Medicare beneficiaries newly diagnosed with VTE with and without OAC treatment within the month from first VTE and identify factors associated with being untreated in that period. Additionally, to identify factors associated with treatment with DOACs or warfarin among OAC-treated patients.
Methods
Data from 1/1/2014-12/31/2019 of Medicare beneficiaries aged ≥65 years with a new diagnosis of VTE in the inpatient or ambulatory (including emergency room [ER]) setting from 1/1/2015 to 12/31/2019 were included. Additional eligibility criteria included continuous Medicare Parts A, B and D enrollment ≥12 months before and ≥30 days after first VTE diagnosis (index VTE), no diagnosis for atrial fibrillation, inferior vena cava filter, pregnancy or cancer, and no OAC treatment prior to index VTE.
VTE patients with ≥1 outpatient pharmacy claims for a DOAC or warfarin within 30 days from index VTE were considered OAC-treated; otherwise, they were classified as untreated. Patients in the OAC-treated cohort were further classified into the DOAC or warfarin subgroups based on the first OAC treatment observed from index VTE (index OAC). Factors associated with being untreated with OACs (vs. treated) and with receiving treatment with DOACs (vs. warfarin) were assessed using multivariable logistic regressions.
Results
A total of 169,928 patients (49.7% OAC-treated; 50.3% untreated) were identified. In the OAC-treated cohort, 62,528 (74.0%) had DOACs (mainly apixaban [51.9%] and rivaroxaban [46.9%]), and 29,324 (26.0%) warfarin as index OAC.
Within the OAC-treated and untreated cohorts respectively, mean age was 76.2 and 77.3 years, 62.9% and 66.4% were female, 82.6% and 75.4% were Non-Hispanic White, mean CCI score was 2.0 and 3.0, 51.8% and 70.3% had DVT only, and 48.2% and 29.7% had PE.
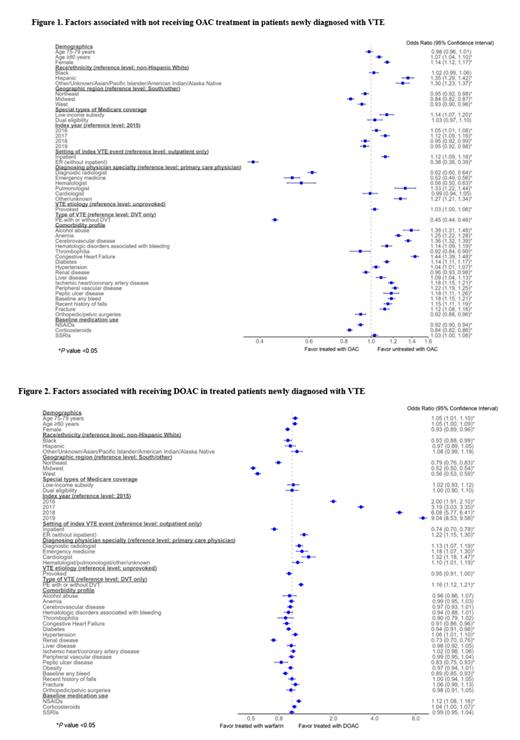
Factors associated with being untreated with OACs (odds ratio; 95% confidence interval) included Hispanic ethnicity (vs. White) (1.35; 1.29-1.42), congestive heart failure (1.44; 1.39-1.48), cerebrovascular disease (1.36; 1.32-1.39), anemia (1.25; 1.22-1.28), and peripheral vascular disease (1.22; 1.19-1.25). Having part D low-income subsidy was also associated with being untreated with OACs (1.14; 1.07, 1.20). Index VTE diagnosis setting being ER (vs. outpatient) (0.38; 0.36-0.39) and pulmonary embolism (vs. DVT) (0.45; 0.44-0.46) were associated with increased odds of being treated with OAC, as was being diagnosed by a radiologist (0.62; 0.60-0.64), emergency medicine physician (0.52; 0.49-0.56) or hematologist (0.56; 0.50-0.63) (vs. primary care) ( Figure 1).
Among the OAC-treated cohort, factors associated with being treated with DOACs vs. warfarin included diagnosis in more recent years, VTE in ER setting (1.22; 1.15-1.30). VTE in inpatient setting (0.74; 0.70-0.78) and renal disease (0.73; 0.70-0.76) were associated with increased odds of being treated with warfarin, as were Northeast (0.79; 0.76-0.83), Midwest (0.52; 0.50-0.54), and West US census regions (0.56; 0.53-0.59), respectively ( Figure 2).
Conclusions
In this study of Medicare patients, nearly half of the patients with ICD codes for VTE were not treated with OAC in the first month from initial diagnosis. Among OAC-treated patients, a higher proportion of patients were treated with DOAC vs. warfarin, consistent with more recent guideline recommendation. Factors associated with being untreated might reflect challenges associated with VTE management among disadvantaged populations and in the presence of certain comorbidities.
Disclosures
Coons:Pfizer Inc.: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding. Wang:Bristol Myers Squibb: Consultancy, Research Funding; Pfizer Inc.: Consultancy, Research Funding. Latremouille-Viau:Bristol Myers Squibb: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.; Pfizer Inc.: Consultancy, Research Funding; Analysis Group, Inc.: Current Employment, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.. Russ:Pfizer Inc.: Current Employment. Cheng:Bristol Myers Squibb: Current Employment. Stellhorn:Bristol Myers Squibb: Current Employment. Dai:Pfizer Inc.: Current Employment. Steffen:Bristol Myers Squibb: Consultancy, Research Funding; Pfizer Inc.: Consultancy, Research Funding. Zion:Pfizer Inc.: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding. Deeba:Pfizer Inc.: Current Employment. Hines:Pfizer Inc.: Current Employment.